Abstract
KMT2C is a haploinsufficient tumor suppressor gene that is recurrently deleted in myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) in the context of larger chromosome 7 deletions. It encodes MLL3, a histone methyltransferase in the COMPASS complex that binds enhancers and promotes gene expressions. We previously reported that Kmt2c deletions enhance hematopoietic stem cell (HSC) self-renewal capacity and convey a selective advantage as HSCs cycle following chemotherapy or transplantation.
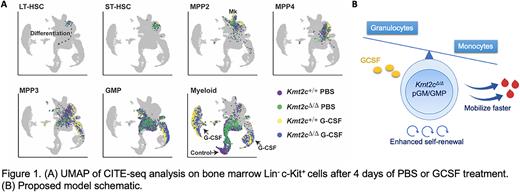
Our prior findings raised the question of whether granulocyte colony-stimulating factor (G-CSF), a cytokine commonly used to expedite neutrophil recovery after chemotherapy and to mobilize HSCs for transplantation, could further select for KMT2C-deleted HSCs. If so, G-CSF could potentiate therapy-related MDS and AML. To address this question, we treated Kmt2cf/f; Vav1-Cre and Kmt2cf/+; Vav1-Cre mice with G-CSF and assessed HSC mobilization to peripheral blood and spleen after 2 and 4 days. Kmt2c-deleted HSCs mobilized faster in response to G-CSF than wildtype HSCs, and this was accompanied by a profound increase in myeloid output in the Kmt2c-deleted, G-CSF-treated mice. Indeed, pre-granulocyte-monocyte progenitor (pGM) and granulocyte-monocyte progenitor (GMP) populations mobilized and expanded rapidly in overall numbers in Kmt2c-deleted mice, though this expansion did not reflect increased proliferation. Strikingly, we found that enhanced HSC mobilization in Kmt2c-deleted mice occurred via non-cell autonomous mechanisms whereas enhanced myeloid expansion was a cell autonomous phenotype. This raised two mechanistic questions: 1) How does Kmt2c/MLL3 restrict myeloid progenitor expansion in response to G-CSF? 2) How might these changes non-cell autonomously influence HSC mobilization?
To understand how Kmt2c/MLL3 deletion regulates myeloid progenitor fate, we performed Cellular Indexing of Transcriptomes and Epitopes by sequencing (CITE-seq) on Lin- c-Kit+ cells with surface barcodes that could resolve HSC, multipotent progenitor (MPP) and myeloid progenitor identities (Figure 1A). Kmt2c deletions enhanced expression of neutrophil-associated genes from the myeloid-biased MPP3 stage onward in differentiation, while it simultaneously reduced expression of genes associated with monocyte and dendritic cell differentiation. Further analysis showed that macrophages and conventional dendritic cells (cDCs) were indeed reduced in Kmt2c-deleted mice, particularly after G-CSF treatment. Thus, MLL3 tunes granulocyte and monocyte/dendritic cell output from a common progenitor such that enhanced granulocyte production comes at the expense of monocyte/dendritic cell production. Of note, bone marrow macrophages and cDCs have previously been shown to limit permeability of the bone marrow endothelium and suppress HSC mobilization. A reduction in macrophage/cDCs in Kmt2c-deleted mice could non-cell autonomously enhance HSC mobilization.
Furthermore, we performed culture experiments to assess colony formation, cell production and differentiation from Kmt2c-deleted pGMs and GMPs. We found that both populations expanded to a greater extent, and maintained undifferentiated morphologies for longer periods of time, than their wildtype counterparts. These differences were again amplified by G-CSF. Though self-renewal capacity in pGMs and GMPs remained limited, loss of MLL3 extended the number of self-renewing divisions that these cells could undergo.
Altogether, our data show that MLL3 balances the granulocyte/monocyte cell fate decisions in myeloid progenitors, and it restricts self-renewal capacity (Figure 1B).
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal